Gelacell™ 3D nanofibrous scaffold for cell culture and tissue engineering
3D culture with nanofibrous scaffolds
In the past decades, the two-dimensional culture approach has been the standard for any research involving the culture of eukaryotic adherent cells. This approach is extensively documented and well characterized; however, it suffers from major drawbacks which stem primarily from the adaptations that cells must undergo in a 2D culture approach. Cells in a 2D culture environment must flatten and stretch into a monolayer to adhere and grow on the surface. This drastically alters their shape and gene expression, limits their cell-to-cell interactions, and modifies their natural responses to external stimuli. Thus, the biological relevance of research performed on a 2D monolayer is limited by its inability to reproduce the complex behavior found in native tissues. That is why in recent years there has been a marked effort towards the use of 3D culture systems that can better resemble living tissue conditions [1].
Whether the research involves oncological studies, toxicity screening, tissue engineering for regenerative medicine, ECM interactions, and complex systems modeling, or efficiency assessment of chemical compounds for novel therapeutics, it is crucial to perform experiments in environments that closely resemble the structure of a natural extracellular matrix to improve the accuracy and relevance of the results.
Among various technology platforms supporting 3D cell culture, nanofibrous scaffolds offer several unique benefits that make them stand out as a superior alternative. Namely, the high surface-to- volume ratio, the variety of its possible polymeric components, the tunability of their mechanical and surface chemical properties, their high porosity that allows nutrients transfer and cell motility, and their ease for scalable standardized production [2]. All these elements make nanofibrous scaffolds an ideal candidate to act as an in-vitro ECM with exceptional performance.
Concurrently, and especially regarding tissue engineering applications, obtaining scaffolds that mimic the tissue native architecture at the nanoscale has always been a major challenge, specifically the reproduction of the interwoven nanostructure created by natural ECM, which supports and directs cell adhesion, proliferation, and bioactivity.
The use of Gelacell™ nanofiber scaffolds can address these challenges by providing a porous and highly interconnected fibrous network that can act as an unparalleled substitute for the native microenvironment, providing a suitable material for cellular compatibility, tissue engineering, drug discoveries, regenerative medicine, and other relevant cell-biological applications.
Description of Gelacell™ nanofibrous scaffold
Gelacell™ 3D nanofibrous scaffold is a non-woven highly porous scaffold specially designed for in vitro 3D cell culture and tissue engineering. The products are uniquely created by utilizing polymers that are frequently employed in cell culture-related research, facilitating their application in more complex cell culture studies. The product portfolio of GelacellTM scaffolds is briefly illustrated in Figure 1 and Table 1.
Gelacell™ scaffolds are majorly composed of either natural or synthetic polymers. The products containing natural biopolymers such as gelatin and chitosan were made by crosslinking the protein amino groups present in gelatin molecules, forming covalent bonds that create a network structure. The degree of crosslinking was finely tuned to preserve the essential properties of gelatin, such as mechanical strength, absorption capacity, resistance to enzymatic degradation, and bioactivity (cell-binding domains or growth factor binding sites). The synthetic scaffold variants contain PLLA, PLGA, PLGA:PCL, and PHB. These polymers are well suited due to their biocompatibility, biodegradability, non-toxicity, batch-to-batch consistency, and reproducibility, compatible with many cell lines, and suitable for the culture of complex systems.
Gelacell™ nanofibrous scaffolds use pharmaceutical grade polymers to ensure high purity, strict control over impurities and low endotoxin levels. The products have been optimized to provide chemical, thermal, and mechanical stability for 3D cell culture, as well as an adequate swelling capability and porosity to allow for nutrient diffusion and avoid cellular waste build-up.
| Application | Product | Material | Device |
|---|---|---|---|
| Short / medium term 3D culture | Well plate | PLLA, PLLA aligned | 24 well plate, scaffold on PET discs |
| Inserts | PLGA, PLGA:PCL, PLLA, PLLA aligned, Gelatin, Chitosan etc | Simple disc inserts | |
| Long term culture | Well plates with cell crowns | PLLA, PLLA aligned, PLGA, PLGA:PCL, Gelatin | 6, 12, 24 well plates with cell crowns |
| Tissue engineering / organoid scaffolds | Sheet of scaffold | As above | 10 cm x 10 cm sheet |
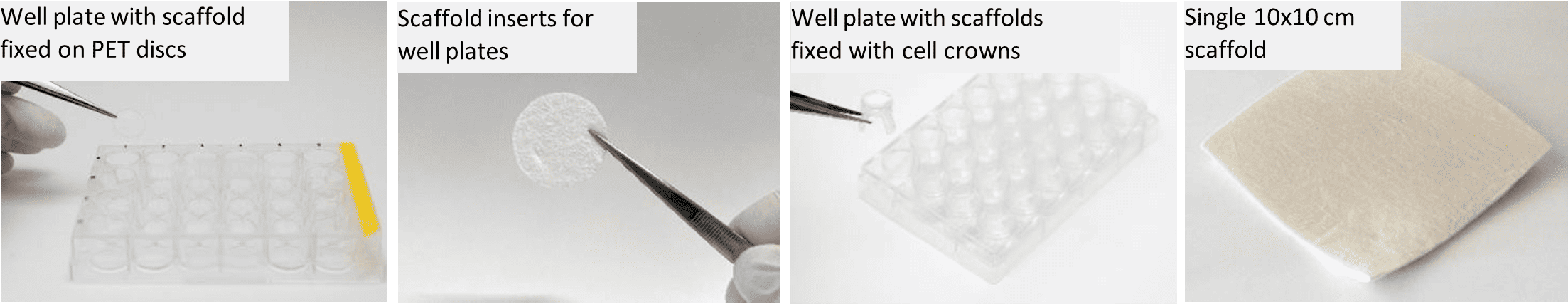
Figure 1: Gelacell™ products portfolio
Advantages of Gelacell™ nanofibrous scaffold
Gelacell™ scaffolds come with several advantages that are inherent to the proprietary patented HaloSpin™ technology, ultimately producing a 3D microarchitecture fundamentally different from the planar and stiff alignment surfaces of alternative nanofiber production methods like electrospinning. Gelacell™ nanofibers form a unique porous network which provides a 3D framework for an in vitro ECM analog suitable for the culture of many cell types such as fibroblasts, myoblast, osteoblast, MSCs, iPSCs, liver cells, chondrocytes, neural cells, etc.
In Figure 2, SEM pictures of two different scaffolds produced using halospinning technology are represented. The morphological advantages of nanofibers are depicted in the SEM images. The high surface area-to-volume ratio provides more sites for cell adhesion, proliferation, and interaction, facilitating improved cell-material interactions and biological responses. The nanoscale dimensions and fibrous morphology of nanofibers more closely resemble the native ECM. The tunable properties of fiber diameter and porosity allow to create scaffolds with specific features that can influence cell behavior, nutrient transport, and tissue ingrowth. These aforementioned characteristic features describe the fabrication of a 3D architecture by halospinning technology, that provides a more biomimetic environment for cells by allowing them to grow in a three- dimensional space, promoting cell proliferation, differentiation, and tissue formation.
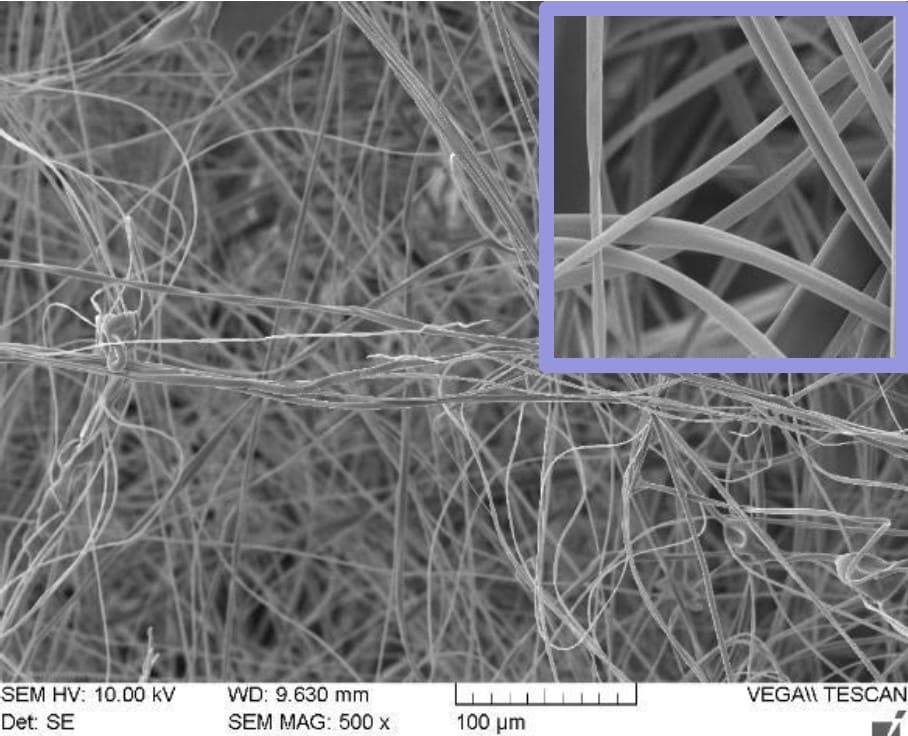
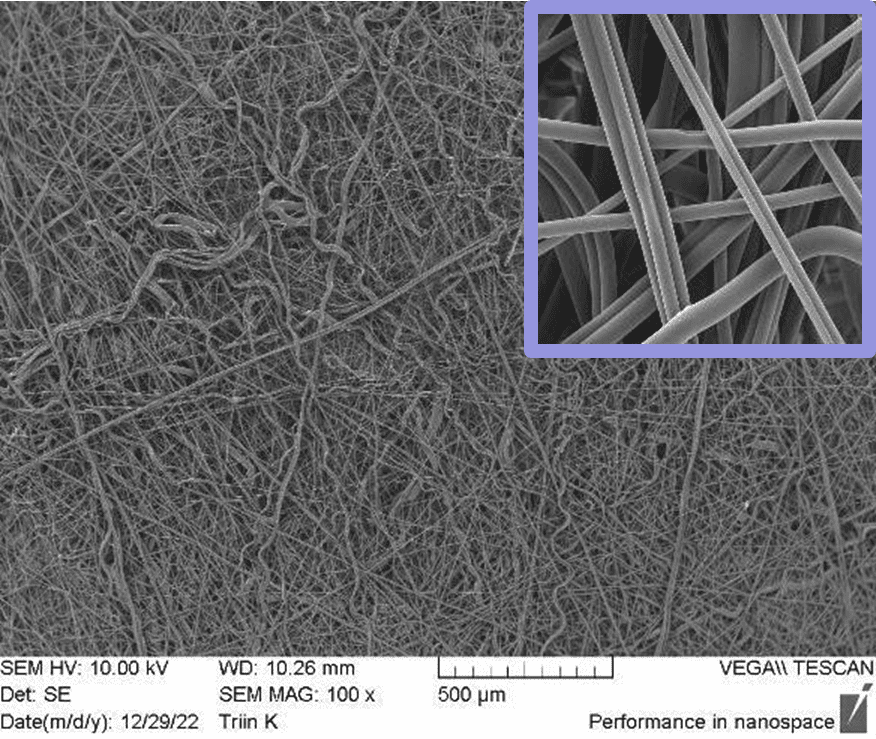
Figure 2: SEM images of Gelacell™ Gelatin (left) and PLGA (right) scaffold demonstrating the 3D nanofibrous structure.
The HaloSpin™ technology used to manufacture Gelacell™ 3D nanofibrous scaffolds provides a standardized, easily scalable, high-throughput production process, with batch-to-batch reproducibility, at a lower cost when compared with electrospinning.
The scaffold maintains its viability for extended periods of time when stored under dry conditions at room temperatures.
Gelacell™ products are packaged in adherence to ISO Class 7 clean room conditions and sterilized using gamma radiation.
Gelacell™ product performance
The following section illustrates two case studies carried out with Gelacell scaffolds. The first study examines cellular behaviors in relation to scaffolds made from a natural polymer (gelatin) and the second study in relation to scaffolds made from a synthetic polymer (PLGA).
Case study 1: Cell culture studies on a natural biopolymer (gelatin)
BHK21 Cell viability and visualization after 24 hours
To evaluate the performance of the gelatin scaffold in comparison with a standard nitrocellulose membrane (NC), and a traditional 2D culture at the bottom of the well (2D well), cell viability assays, and confocal microscopy observations were performed after 24 hours. Fibroblast cells (BHK21) were seeded for this purpose. Two distinct seeding concentrations were employed for the MTS assays, while a single seeding density was used for confocal microscopy on two identical gelatin scaffolds (gelatin scaffold 1 and 2). The detailed methodology of the tests can be found in the Methodology section of this report.
Figure 3 depicts the results of the MTS cell activity assay after a 24-hour period across three distinct platforms and various seeding concentrations. This could be attributed to the superior cellular adherence profile of the gelatin scaffold in comparison to the nitrocellulose membrane.
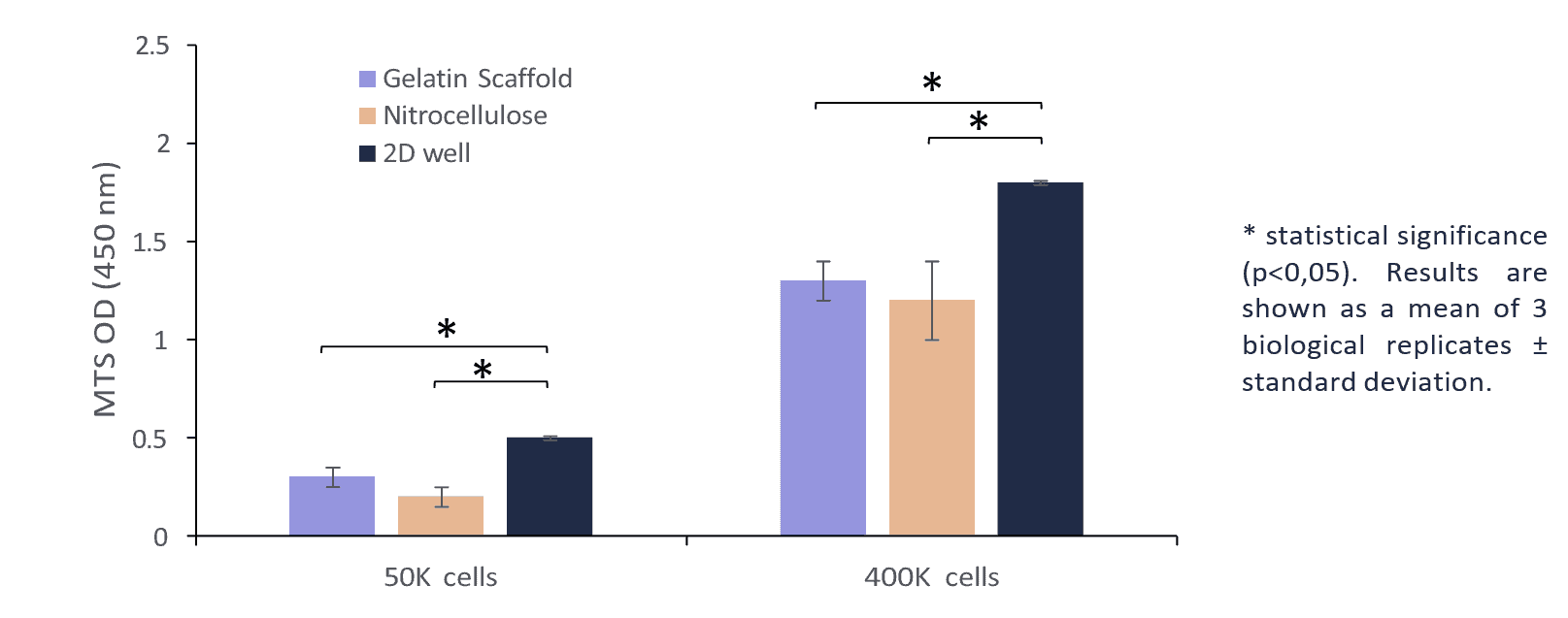
Figure 3: Viability of BHK 21 cells on Gelacell™ gelatin scaffold, nitrocellulose membrane and 2D well plate after 24 h of incubation using different cell seeding concentrations. Cells on the bottom of 24 well plates and nitrocellulose were used as controls.
Confocal microscopy was performed on cells stained with DAPI and Alexa 568 following 24 hours of incubation. The main objective of these observations was to determine the structural differences between the cells that had been incubated in the 3D environment of the gelatin scaffold, and the cells that had grown into the traditional 2D monolayer at the bottom of the well. Examining the structural changes and growth patterns of the cells in these different conditions provides insights into the feasibility of using the gelatin scaffold as a suitable growth substrate for eukaryotic cells.
Figure 4 depicts confocal images of viable cells in the gelatin scaffold, adhered to nanofibers, and compares them with the cells in the 2D control. Figure 5 shows the 3D render of a confocal image Z- stack shows cells at different depths of the gelatin scaffold.
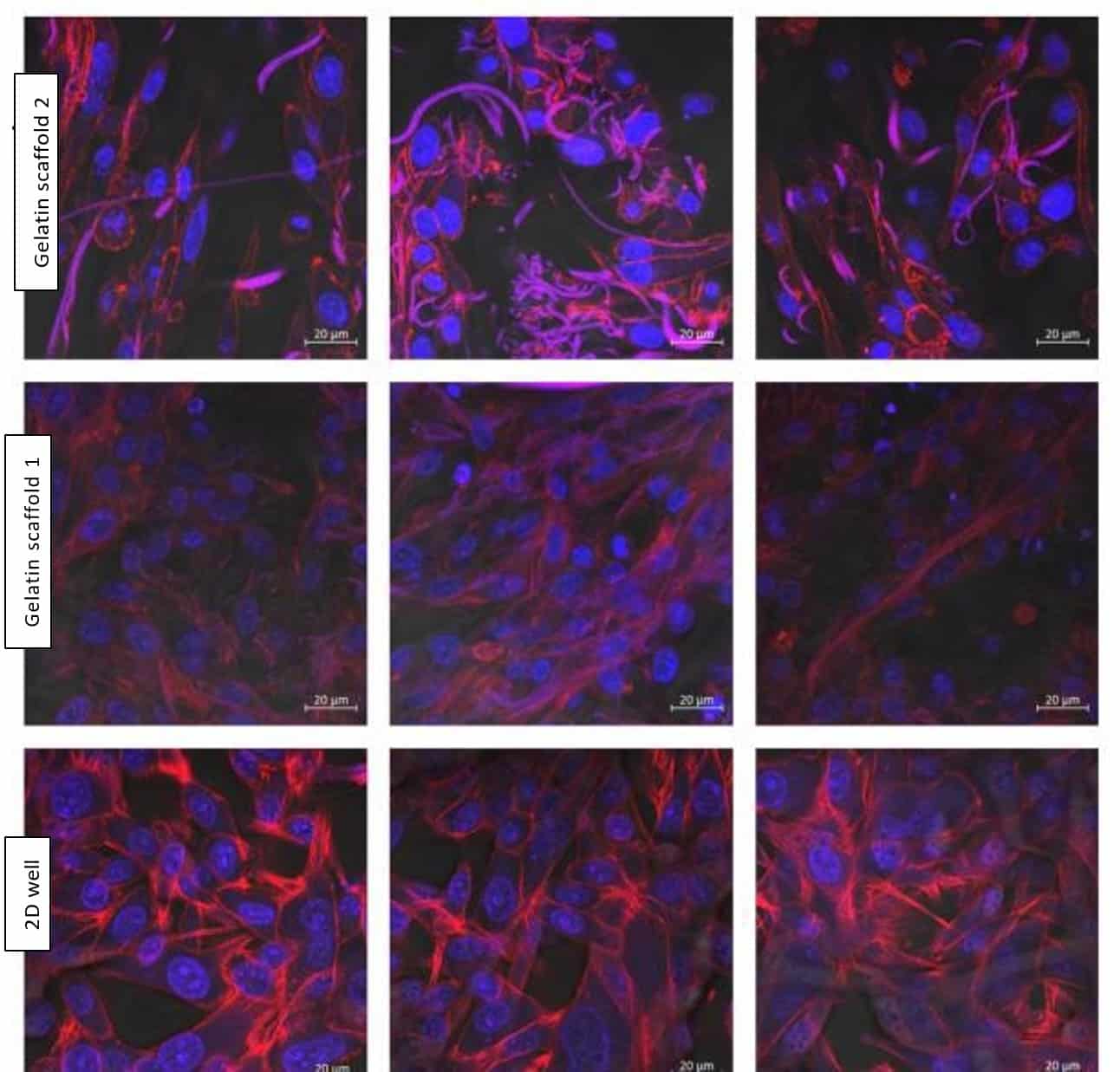
Figure 4: Confocal microscopy images. Cell structure after growing on the Gelacell™ gelatin scaffolds for 24 h and using an initial seeding concentration of 400 000 cells per well. Images from different locations within the sample are depicted in the same row. Blue – DAPI stained cell nucleus. Red – Alexa 568 stained actin filaments. Images show that fibers also exhibit autofluorescence (represented as pink-purple). The control is the cells grown for 24 hours on a 2D layer at the bottom of the well.
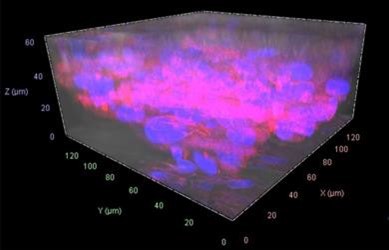
Figure 5: Z-stack 3D image showing cell (BHK21) locations in different planes within the Gelacell™ gelatin matrix (400 000 cells per well after 24 hours).
Figures 4 and 5 together demonstrate successful cell motility within deeper layers of the gelatin scaffolds, reflecting the cells’ capacity to traverse, migrate inward, and evenly distribute within the matrix. This is mainly believed to be due to the intrinsic highly porous and flexible 3D structure of the nanofibrous network inherent of the GelacellTM scaffold.
When compared to the control set, the actin filaments prevalence of the cells cultured in the Gelacell™ scaffold is less noticeable, hinting that the cells do not need to undergo harsh morphological changes in order to improve their adherence to the surface. The nanofibrous morphology of gelatin and presence of bioactive sites provides a high attachment and spreading of fibroblast cells. The surface topography of the nanofibers, such as fiber diameter, alignment, and smoothness, impacted higher cell adhesion. The degree of gelatin crosslinking did not impact the accessibility of cell-binding motifs within the gelatin scaffold. It is believed that, aside from a support network, the nanofibrous structure of the scaffold provides mechanosensory feedback for the cells that is more similar to that of a naturally occurring tissue. This idea is further supported by the observation of the flat cell morphologies of the control sample and comparing them with the more elongated shape of the cells grown on the Gelacell™ scaffolds.
Cell viability and visualization after 1, 3, and 7 days
The cell proliferation and growth kinetics on the gelatin scaffold in comparison with 2D culture at the bottom of the well (2D well) were evaluated by culturing BHK21 cells for 1, 3, and 7 days. The seeding concentration for all samples was 100k cells per well. The detailed method of the tests can be found in the Methodology section of this report.
Figure 6 shows the results of the cell viability CCK8 assays for all samples throughout the 7-day period. A significant difference in cell growth was detected between the two platforms at the measured times. In the specific case of the gelatin scaffold, it was observed that after 7 days, it had the highest cell viability measurement compared to the cells cultured on the bottom of the well (2D well). These results suggest that the 3D nanostructure of the GelacellTM scaffold, with its morphological advantages and porous structure, greatly enhances cell growth and viability.
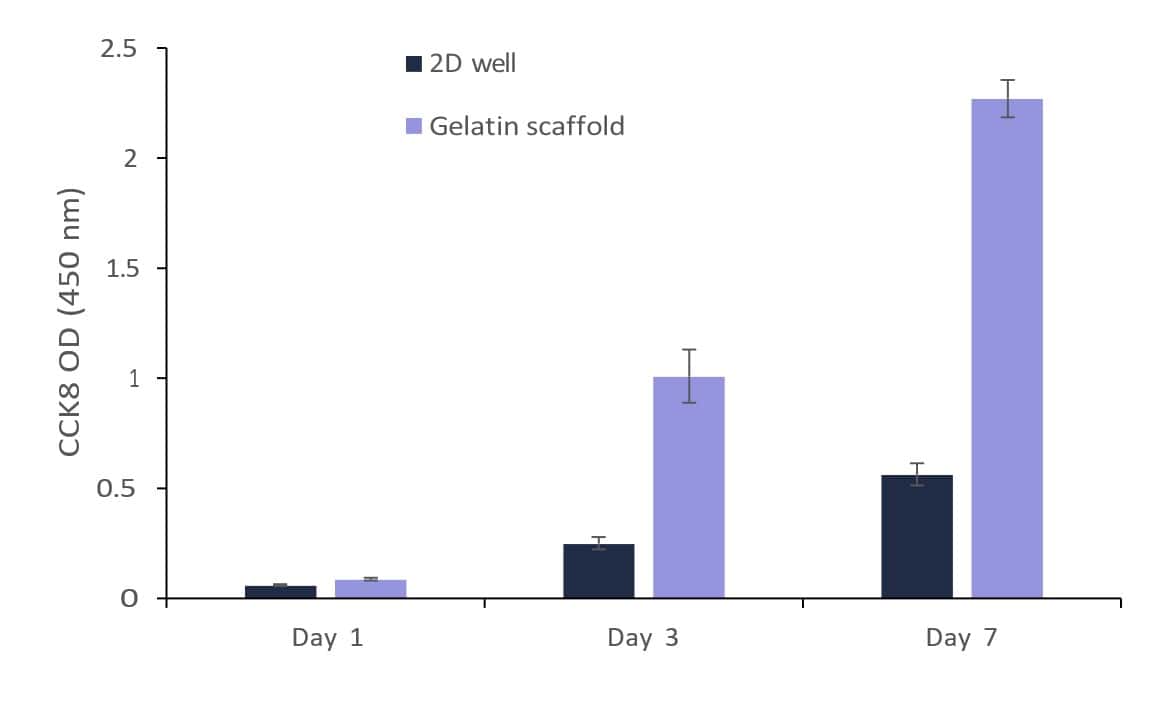
Figure 6: Viability of cells at different time-points (1, 3, and 7 days) after seeding of BHK21 cells on Gelacell™ gelatin scaffolds and at the bottom of the well plate (2D well) (n=3; error shows the standard deviation).
For the confocal microscopy, the cells were stained with DAPI and Alexa 568 after 1, 3, and 7 days of incubation. Again, the main objective of these observations was to determine the structural differences between the cells that had been incubated in the 3D environment of the Gelacell™ scaffold, and the cells that had grown into the traditional 2D approach at the bottom of the well after a week of culture.
A visual inspection of the wells at the 72-hour mark revealed that the cells seeded on the bottom of the well were reaching a high level of confluency and the first instances of multi-layering occurred. This phenomenon continued until the seventh day of the experiment as cells kept expanding as indicated by the CCK8 assay results.
The images resulting from the confocal microscopy observations for the 1, 3, and 7 days can be seen in Figure 7, Figure 8, and Figure 9 respectively.
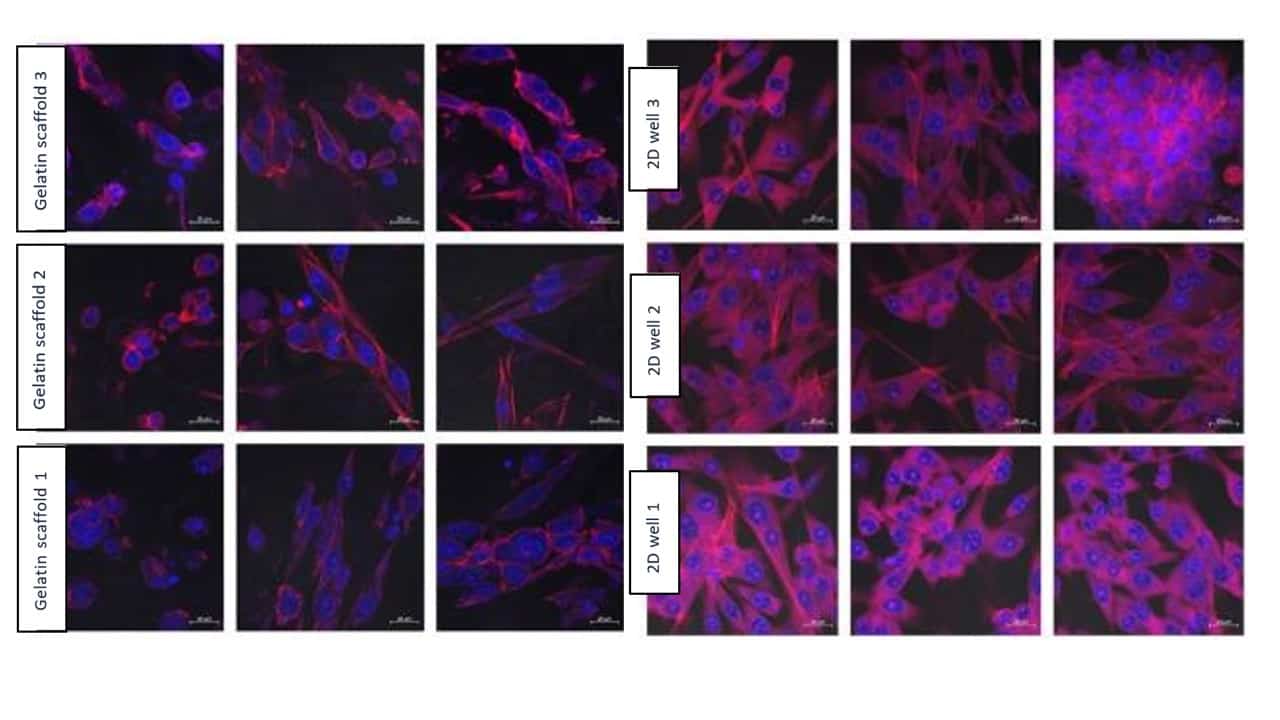
Figure 7: Results from confocal microscopy after 1 day, comparing Gelacell™ gelatin scaffolds and 2D wells. Cell (BHK21) seeding concentration was 100 000 per well. Measurements from the same samples are shown in one row. Blue – DAPI stained nucleus of the cell. Red – Alexa 568 stained actin filaments
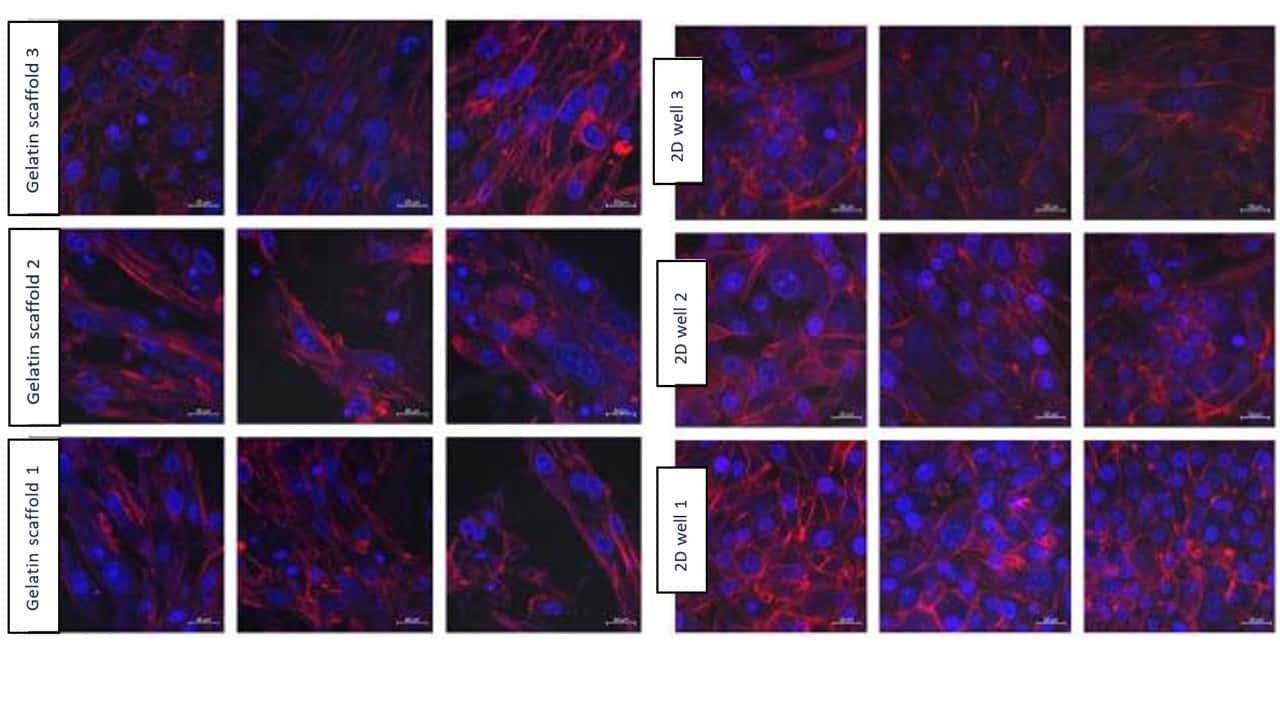
Figure 8: Results from confocal microscopy after 3 days, comparing Gelacell™ gelatin scaffolds and 2D wells. Cell (BHK21) seeding concentration was 100 000 per well. Measurements from the same samples are shown in one row. Blue – DAPI stained nucleus of the cell. Red – Alexa 568 stained actin filaments
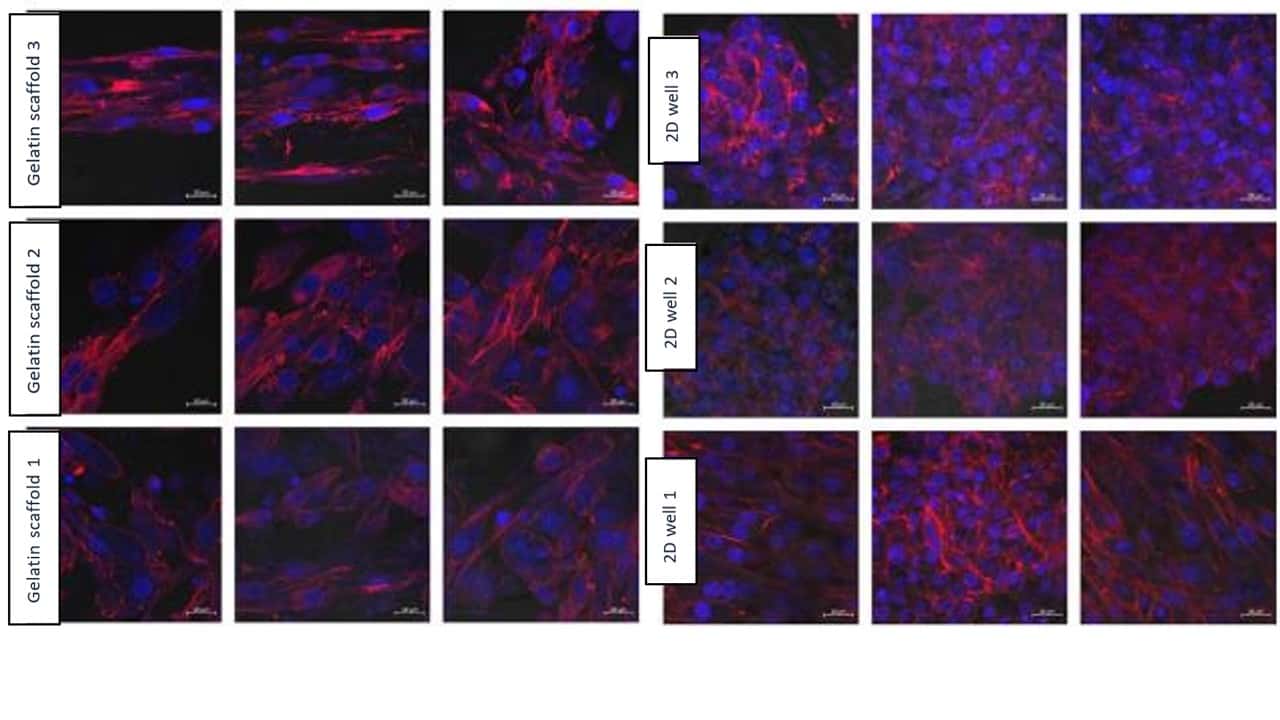
Figure 9: Results from confocal microscopy after 7 days, comparing Gelacell™ gelatin scaffolds and 2D wells. Cell (BHK21) seeding concentration was 100 000 per well. Measurements from the same samples are shown in one row. Blue – DAPI stained nucleus of the cell. Red – Alexa 568 stained actin filaments
When comparing Figures 7 to 9, it can be noticed that the highest number of cells visualized in the gelatin scaffold increased from the 24-hour mark to its maximum at 72 hours, coinciding with the time point where layering started to occur in the cells seeded at the bottom of the well. After 7 days, fewer cells were visually observed at the GelacellTM scaffold superior layers. This is believed to be caused by the migration of cells inside the scaffold and the limitations of the visualization method to penetrate deeper into the matrix layers. This inference is supported by the increase of the OD measured on the CCK8 assay for the gelatin scaffold during the experimental period.
C2C12 Cell viability and visualization after 1, 3, and 7 days
The gelatin nanofibrous scaffold was also utilized to culture myoblast cells (C2C12). A pre-test conducted on the gelatin scaffolds over 24 hours allowed us to predetermine the cell compatibility and seeding density. The results confirm successful adhesion and myoblast compatibility on the gelatin scaffolds. The seeding density was optimized according to WST activity from the 24-hour test. Later, the myoblast culture was extended to 7 days. Figure 10 shows the images of the cells (C2C12) cultured after 1, 3, and 7 days. Calcein and propidium iodide were used to stain and distinguish the live and dead cells on the scaffolds.
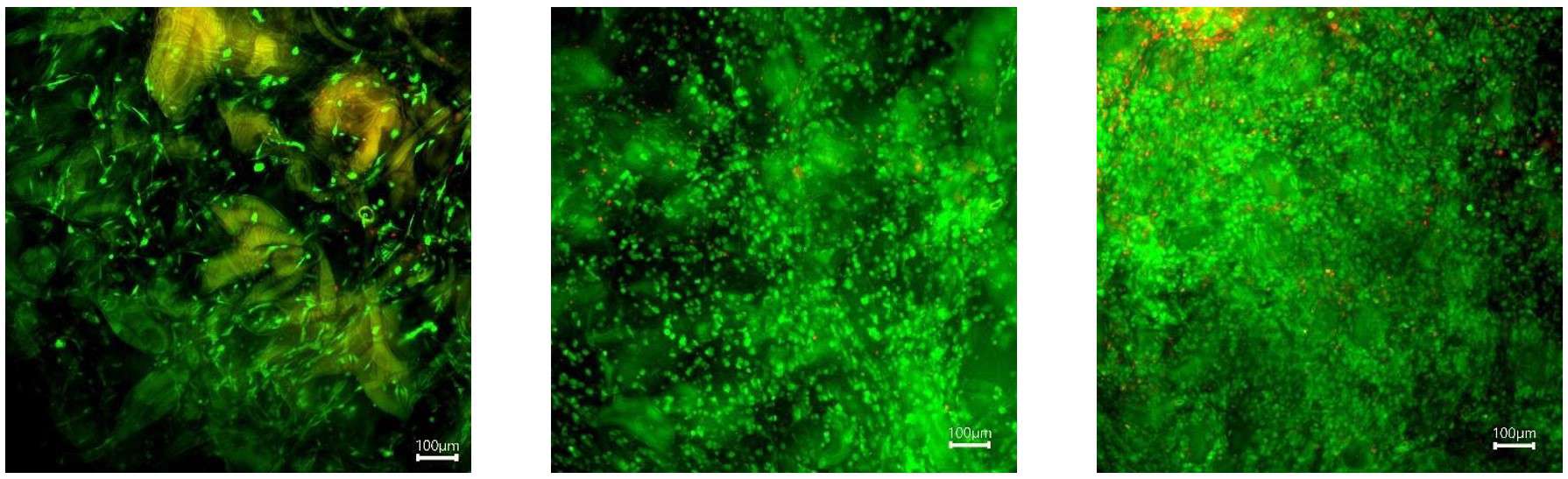
Figure 10: Myoblast (C2C12) stained with calcein (live) and PI (dead) on Gelacell™ gelatin scaffolds after 1, 3, and 7 days (left to right) of culture.
Gelatin nanofiber scaffolds provide a favorable surface for myoblast adhesion due to its fibrous structure and the presence of cell-binding motifs within gelatin [3]. The nanofiber morphology offers a large surface area for cell attachment, allowing myoblast to interact with the scaffold’s topography and adhere to the nanofiber surfaces. The integrin receptors on the cell membrane engage with the available RGD (Arg-Gly-Asp) sequences and other cell-adhesive ligands present in gelatin, promoting cell adhesion. The fiber orientation provided physical cues that guide myoblast movement along a preferred direction, mimicking the natural alignment of muscle tissue. Myoblasts can extend filopodia and lamellipodia along the nanofiber surfaces, facilitating their migration through the scaffold. Fiber alignment and spacing can influence the speed and directionality of myoblast migration. The scaffolds offer an environment conducive to myoblast proliferation. The high surface area-to-volume ratio of the nanofiber structure allows for cell attachment and facilitates nutrient and oxygen diffusion throughout the scaffold. The presence of bioactive moieties within gelatin, coupled with the mechanical properties of the scaffold, can promote myoblast proliferation and support the formation of myotubes, which are the initial steps towards muscle tissue development.
The cellular behavior of two different cell lines (BHK21 and C2C12) confirms and greatly supports the benefits of GelacellTM nanofibrous scaffold made from natural polymers. The gelatin scaffold provides a richer environment for cellular interaction and supports a network in which the cells do not need to greatly modify their morphological confirmation to improve their adhesion and enable their motility. Gelatin nanofiber scaffolds can stimulate cells to produce and deposit ECM components. As cells adhere and proliferate on the nanofiber surfaces, they can actively synthesize and secrete ECM proteins, such as collagen and fibronectin, contributing to tissue regeneration and scaffold integration. This ECM production helps to create a favorable microenvironment for cell growth and tissue formation. The cell adhesion, migration, and proliferation on the gelatin nanofibers shows promising properties which are useful in various applications such as cell culture related investigations, tissue reconstruction, regenerative medicines, and drug screening/discoveries.
Case study 2: Cell culture studies on a synthetic biopolymer (PLGA) of Gelacell™
A range of synthetic polymers, including PLLA, PLGA, PLGA-PCL, and PHB, are also included in Gelacell™ products. These polymers are widely accepted as a suitable biomaterial due to their great versatility and adaptability in cell-biological applications. The features of these polymers, which include mechanical strength, flexibility, biocompatibility, and rate of degradation, were tailored to meet up the demand for 3D cell culture. This section mainly describes the cellular behavior on PLGA nanofibrous scaffolds. The physical properties namely, thickness, swelling, degradation, tensile strength, and elongation were evaluated in-house and the results can be found in the Product Data Sheet (PDS).
PLGA (poly(lactic-co-glycolic acid)) is a copolymer composed of lactic acid and glycolic acid, both of which are naturally occurring compounds. The polymer is formed through the condensation polymerization of lactic acid and glycolic acid. The 50:50 compositions of these two monomers were utilized to produce PLGA scaffolds using halospinning technology. The architecture of the nanofibers (Figure 2) renders the scaffolds useful in cell culture applications due to their superior biocompatibility and biodegradability, and their close resemblance to the structure and composition of the native extracellular matrix (ECM). The fibrous structure mimics the fibrous nature of the ECM, creating a more physiologically relevant environment for cells to grow and interact. Figures 11 and 12 demonstrate that PLGA nanofibers enable the adhesion, proliferation, and migration of two distinct cell types (fibroblast and myoblast) without inducing significant cytotoxicity or immune responses. The detailed method of cell culture is given in the Methodology section of this article.
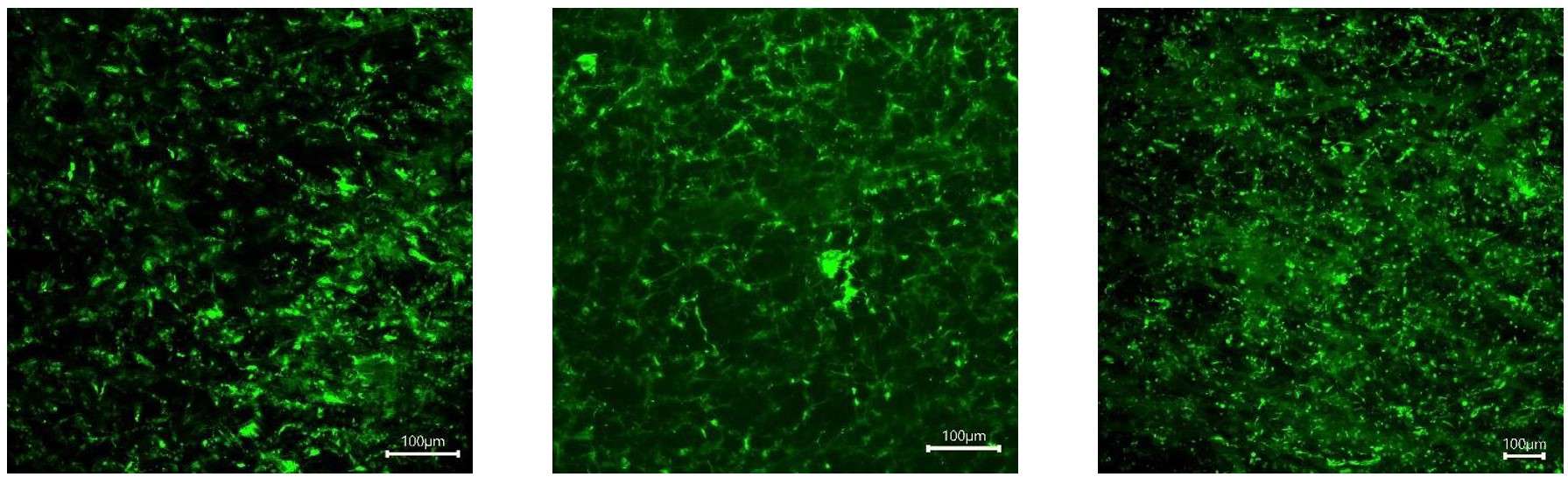
Figure 11: Fibroblast (BHK21) stained with calcein (live) and PI (dead) on Gelacell™ PLGA scaffolds after 1, 3, and 7 days (left to right) of culture.
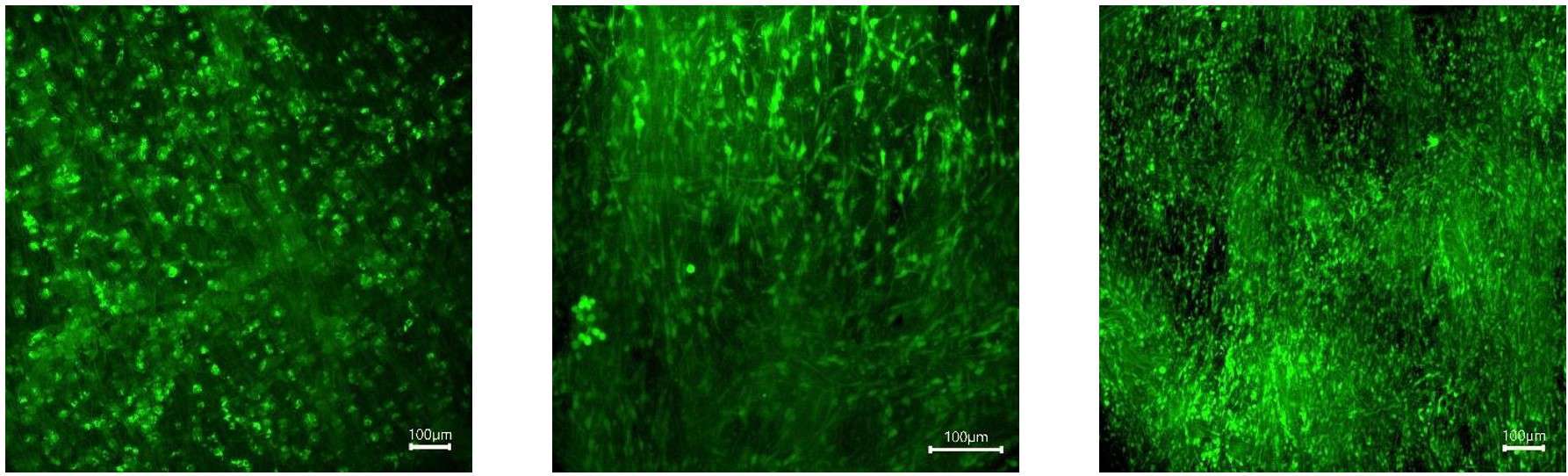
Figure 12: Myoblast (C2C12) stained with calcein (live) and PI (dead) on GelacellTM PLGA scaffolds after 1, 3, and 7 days (left to right) of culture.
The growth of fibroblast and myoblast cells on the PLGA scaffolds can be further appreciated by confirming the CCK8 assay test (Figures 13 and 14). Both cell types showed typical adherence to PLGA nanofiber scaffolds and spread out on the surface. The PLGA nanofiber scaffold provides a 3D structure that enables cells to attach and extend their processes, promoting cell adhesion and spreading. The uniform spreading of cells was observed after 24 hours of cell incubation on the scaffold. The carboxylic acid (-COOH) and hydroxyl (-OH) groups present on the PLGA fibers interact with the fibroblast and myoblast cells. These chemical groups can participate in hydrogen bonding and electrostatic interactions with the cell membrane, facilitating cell adhesion.
When fibroblast and myoblast cells encounter PLGA surfaces, proteins in the surrounding culture medium can adsorb onto the PLGA surface. These adsorbed proteins can include adhesive proteins such as fibronectin, vitronectin, or laminin, which are naturally present in the cell culture medium or secreted by the cells themselves. The adsorbed proteins create a layer known as the “protein corona” on the PLGA scaffold, providing a favorable environment for cell attachment [4]. Further, the cells migrated and infiltrated within the nanofiber scaffold, moving along and between the fibers. The behavior was confirmed when the cell images were captured on and beyond 3 days of culture. The interconnected porous structure of the scaffold facilitates cell migration and allows for cell infiltration throughout the scaffold. Fibroblasts and Myoblasts are responsible for synthesizing and depositing ECM components, such as collagen, elastin, and fibronectin. On PLGA nanofiber scaffolds, these cells possibly deposited their ECM components on or around the fibers, leading to the development of a tissue-like extracellular matrix. PLGA nanofiber scaffold provided a supportive environment for both fibroblast and myoblast proliferation. The results from the CCK8 activity (Figures 13 and 14) assay confirm the progressive growth of both the cells on the scaffolds. Compared to 2D well the cell growth was found to be minimal as the available growth area for the cells was reduced and the cell confluency was reached faster than the nanofibrous scaffolds. The scaffold’s porous structure allows for nutrient and oxygen diffusion, which can support cell growth and proliferation. The presence of PLGA nanofibers can provide structural cues that promote myoblast differentiation and fusion into myotubes. Fibroblasts and myoblasts can communicate with neighboring cells through paracrine signaling. The growth behavior of these cells supports that the secrete soluble factors, (such as for fibroblast: cytokines, growth factors, and chemokines, for myoblast: IGF, TGF-β, and MRFs like MyoD and Myogenin) did not affect the behavior and function of nearby fibroblasts or myoblasts. The presence of PLGA nanofibers can potentially modulate paracrine signaling by influencing the diffusion and localization of these soluble factors.
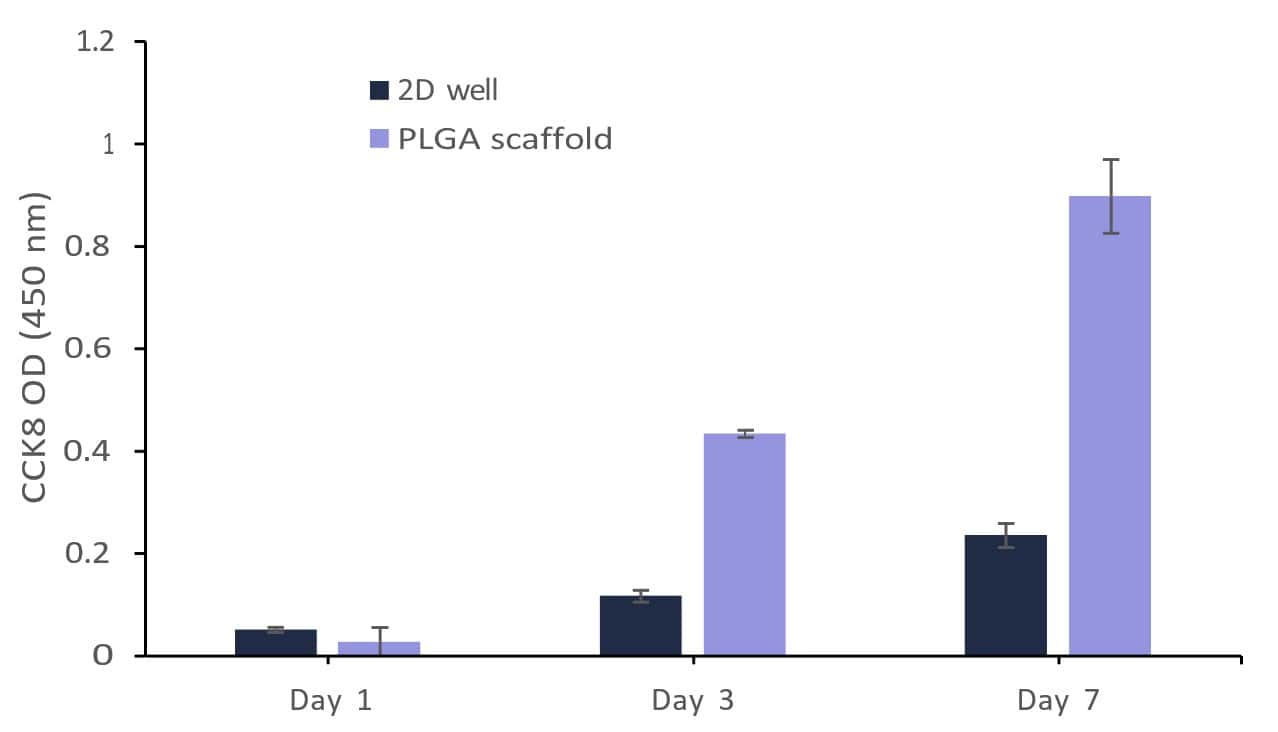
Figure 13: Growth of fibroblast (BHK21) on Gelacell™ PLGA scaffolds in comparison with cells on the bottom of the well plate (2D well).
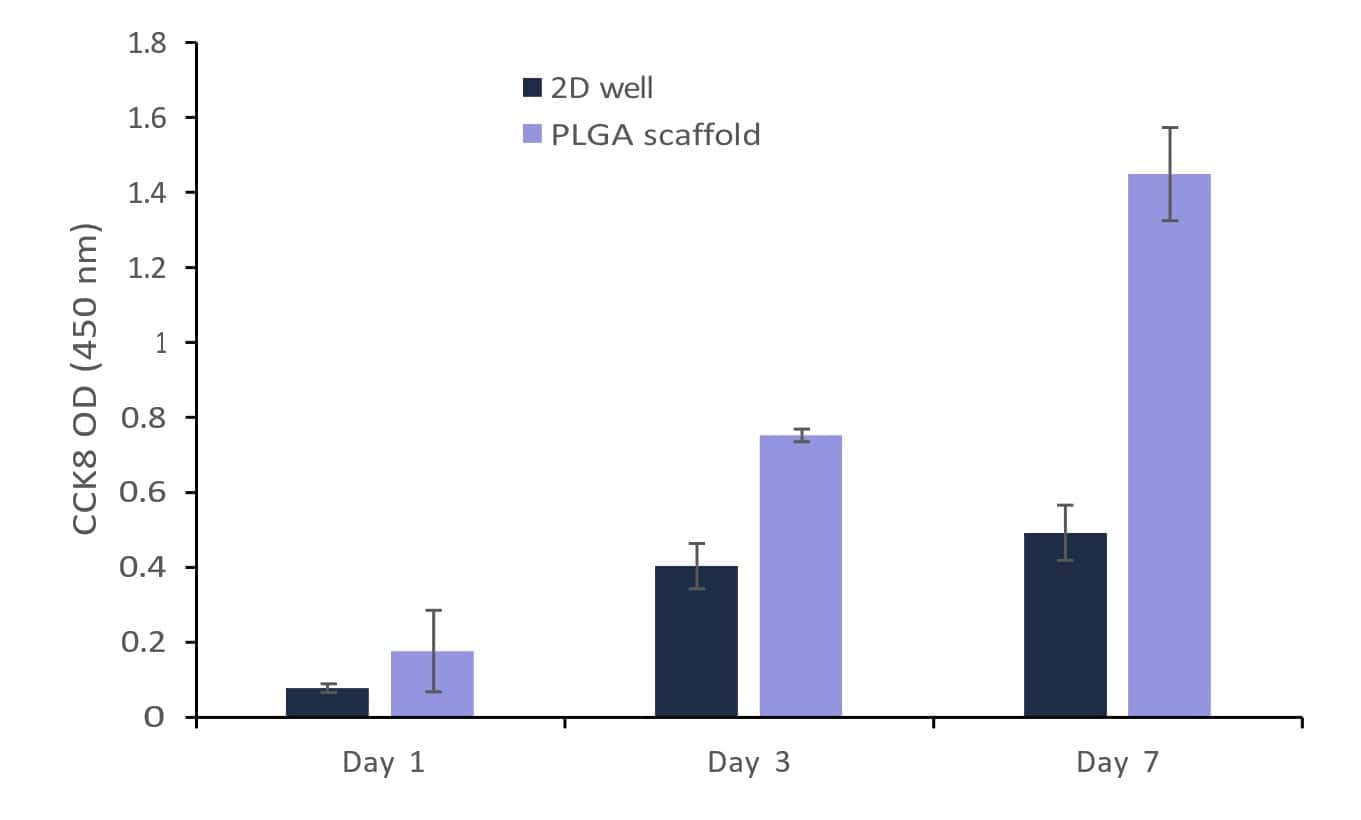
Figure 14: Growth of fibroblast (C2C12) on Gelacell™ PLGA scaffolds in comparison with cells on the bottom of the well plate (2D well).
Similar types of cellular behavior are expected to be observed on the other synthetic polymers (such as PLLA, PLGA-PCL, and PHB) of Gelacell™. As evident from the literature, these polymers are biocompatible, they do not induce toxic or inflammatory responses in cells. They allow cells to attach, proliferate, and maintain their normal functions. These polymers with high biocompatibility are often chemically stable, non-toxic, and non-immunogenic. Functional groups on these synthetic polymeric surfaces can interact with cell membranes and proteins, affecting cell adhesion and signaling. Cells possess charged receptors, and the surface charge of these synthetic polymers can affect the binding and activation of these receptors. All Gelacell™ synthetic products are expected to be positively charged polymers that can promote cell adhesion, migration, proliferation, and differentiations. Therefore, the versatility of synthetic polymers into nanofiber structure can allow researchers to meet specific needs for different cell culture related applications.
Conclusion
In this paper, several experiments provided evidence that the GelacellTM scaffolds are suitable for the 3D culture of eukaryotic cells. The scaffold is biodegradable, biocompatible, and does not present cytotoxicity nor negatively impact cell adhesion, proliferation, or metabolic activity.
It is evidenced that the internal network of interconnected GelacellTM nanofibers creates an adequate environment to facilitate cell migration and proliferation while maintaining lower levels of cellular stress, demonstrated in less morphological deformation and actin activity when compared to a traditional 2D culture approach.
It can be then concluded that the GelacellTM scaffolds mimic, through their composition and morphology, the natural collagenous arrangement of a native extracellular matrix. This in turn promotes cell adhesion, cell motility, and provides an ECM-like support for 3D cell-to-cell, and cell- to-scaffold interactions. Therefore, it is expected that results from cell culture experiments that use the GelacellTM 3D nanofibrous scaffold platform, will better reflect the processes found in natural tissues and might achieve improved biological relevance.
References
[1] Ravi, Maddaly, et al. “3D cell culture systems: advantages and applications.” Journal of cellular
physiology 230.1 (2015): 16-26.
[2] Vasita, Rajesh, and Dhirendra S. Katti. “Nanofibers and their applications in tissue engineering.”
International Journal of nanomedicine 1.1 (2006): 15.
[3] Wang, Chia-Yu, et al. “Polymeric gelatin scaffolds affect mesenchymal stem cell differentiation
and its diverse applications in tissue engineering.” International Journal of Molecular Sciences 21.22
(2020): 8632.
[4] Saltzman, W. Mark, and Themis R. Kyriakides. “Cell interactions with polymers.” Principles of
tissue engineering (2020): 275-293.